Krebs Cycle | Citric Acid Cycle
Krebs cycle is also known as the citric acid cycle was named after the Hans Adolf Krebs who discovered it in 1937. It is also known by several other names such as citric acid cycle, Tricarboxylic Acid Cycle or TCA cycle.
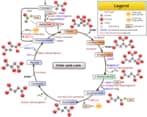
Krebs cycle or citric acid cycle or TCA cycle is a series of biochemical reactions that happen in the mitochondrial matrix. In this reaction, the acetyl portion of acetyl CoA is oxidized to carbon dioxide and the reduced coenzymes FADH2 and NADH are produced. The Krebs cycle is what is known as Amphibolic, in that it is both catabolic (breaks down molecules) and anabolic (builds molecules). It is a series of chemical reactions used by all aerobic organisms to generate energy through the oxidization of acetate derived from carbohydrates, fats, and proteins into carbon dioxide. Therefore
Each stage in the cycle (and in the link reaction – pyruvate conversion into acetyl CoA) occurs twice for every glucose molecule that enters glycolysis because 2 pyruvate molecules are produced for each glucose. Therefore The eight steps of the citric acid cycle are a series of redox, dehydration, hydration, and decarboxylation reactions. Each turn of the cycle forms one GTP or ATP as well as three NADH molecules and one FADH2 molecule, which will be used in further steps of cellular respiration to produce ATP for the cell. Therefore
Learn why 1 million+ medical students and doctors have joined MadeForMedical to master medical sciences.
Click Here: https://www.madeformedical.com/master-bascis-mfm
Unlocking of Terms
Oxidation
Removal of electrons from a molecule is called as oxidation. This subsequently lowers the energy content of a molecule. Most biological oxidations involve the loss of hydrogen atoms. This type of oxidation is referred to as a dehydrogenation and enzymes used for this purpose are called as dehydrogenases. It involves the gain of oxygen atoms. Therefore
Reduction
It is the opposite of oxidation. It is the addition of electrons to a molecule when a molecule is oxidized, the liberated hydride ions (H-) do not remain free in the cell. In order to harness the energy of these electrons, they are immediately transferred to another compound by coenzymes. It is characterized by the loss of oxygen atoms. Therefore thus
- Phosphorylation – It is accomplished by transferring a phosphate group to ADP. Therefore
- Decarboxylation – carbon chain is shortened by the removal of a carbon atom (COO-) as CO2.
- Isomerization – is the process by which one molecule is transformed into another molecule which has exactly the same atoms.
- Dehydration – removing of water molecules. Therefore
- Hydration – the addition of water molecules Therefore
Link Reaction between Glycolysis and Krebs Cycle is the conversion of pyruvate to Acetyl CoA. Pyruvate is transported across the mitochondrion’s inner membrane and into the inner compartment, called the matrix. An enzyme called pyruvate dehydrogenase complex splits each molecule of pyruvate into a molecule of CO2 and a two-carbon acetyl group. Therefore
The CO2 diffuses out of the cell, and the acetyl group combines with a molecule called coenzyme A (abbreviated as CoA). The product of this reaction is acetyl-CoA. • NAD+ is changed to its reduced form, NADH that will enter the Electron Transport Chain. Therefore
Reactions of the Citric Acid Cycle
Step 1: Formation of Citrate
Acetyl CoA, which carries the two-carbon degradation product of glycolysis enters the cycle by combining with the oxaloacetate to give (S)-citryl CoA. The addition is catalyzed by the citrate synthase. (S)-citryl CoA is hydrolyzed to citrate catalyzed by the same citrate synthase to produce CoA-SH and citrate.
Step 2. Formation of Isocitrate
Citrate, a tertiary alcohol, is converted into its isomer, isocitrate, a secondary alcohol, in an isomerization process that involves dehydration followed by hydration that is both catalyzed by the enzyme aconitase. OH, a group from citrate is moved to a different carbon atom.
Step 3. Oxidation of Isocitrate and Formation of CO2 Therefore
This step involves oxidation-reduction (the first of four redox reactions in the Krebs Cycle) and decarboxylation. The reaction catalyzed by isocitrate dehydrogenase is complex:
- Isocitrate is oxidized to oxalo-succinate by NAD+, releasing 2 hydrogen atoms. Therefore
- One hydrogen and two electrons are transferred to NAD+ to form NADH; the remaining hydrogen ion is released Therefore
- The oxalo-succinate remains bound to the enzyme and undergoes decarboxylation, which produces the 5-carbon species α-ketoglutarate.
- This step yields the first molecules of CO2 and NADH in the cycle. Therefore
Learn why 1 million+ medical students and doctors have joined MadeForMedical to master medical sciences.

Click Here: https://www.madeformedical.com/master-bascis-mfm
Step 4: Oxidation of α-ketoglutarate and Formation of CO2 Therefore
This second redox reaction of the cycle involves one molecule each of NAD+, CoA-SH, and α-ketoglutarate. The catalyst is an aggregate of three enzymes called the α-ketoglutarate dehydrogenase complex. Both redox reaction and decarboxylation occur. Three products: CO2, NADH, and the 4-carbon species succinyl CoA. This step yields the second molecule of CO2 and NADH in the cycle.
Step 5: Thioester bond cleavage in Succinyl CoA and Phosphorylation of GDP Therefore
Two molecules react with succinyl CoA – a molecule of GDP (similar to ADP) and a free phosphate group (Pi). The enzyme succinyl CoA synthetase removes coenzyme A by thioester bond cleavage. The energy released is used to combine GDP and Pi to give GTP. Succinyl CoA has been converted to succinate.
Step 6: Oxidation of Succinate Therefore
This is the third redox reaction of the cycle. Succinate is dehydrogenated by FAD catalyzed by succinate dehydrogenase to produce fumarate, a 4-carbon species with trans double bond. The FAD is reduced to FADH2. Therefore because
Step 7: Hydration of Fumarate
The enzyme fumarase catalyzes the addition of water to the double bond of fumarate and is therefore known as the nucleophilic addition. The enzyme is stereospecific, so only the L-isomer of the product malate is produced. Therefore because
Step 8: Oxidation of L-Malate to Regenerate Oxaloacetate
This is the fourth redox reaction of the cycle. A molecule of NAD+ reacts with malate, picking up two hydrogen atoms (oxidation) with the associated energy to form NADH+ H+. This reaction is catalyzed by malate dehydrogenase. The product of this reaction is oxaloacetate that can combine with another molecule of Acetyl CoA, and the cycle can begin again.
Learn why 1 million+ medical students and doctors have joined MadeForMedical to master medical sciences.

Click Here: https://www.madeformedical.com/master-bascis-mfm

I found a lot of these ideas and suggestion very well thought out I also found this care plan suggestions…
I have learn a lot, thank so much to share such a good NCP.
Very good. I thanks my teacher
THis was of tremendous help to me.
THANK YOU SO MUCH. MY WORK MADE EASY AND WELL EXPLAINED.